Molecule of the Month: Arc
An unexpected link between viruses and the brain

Arc forms virus-like structures
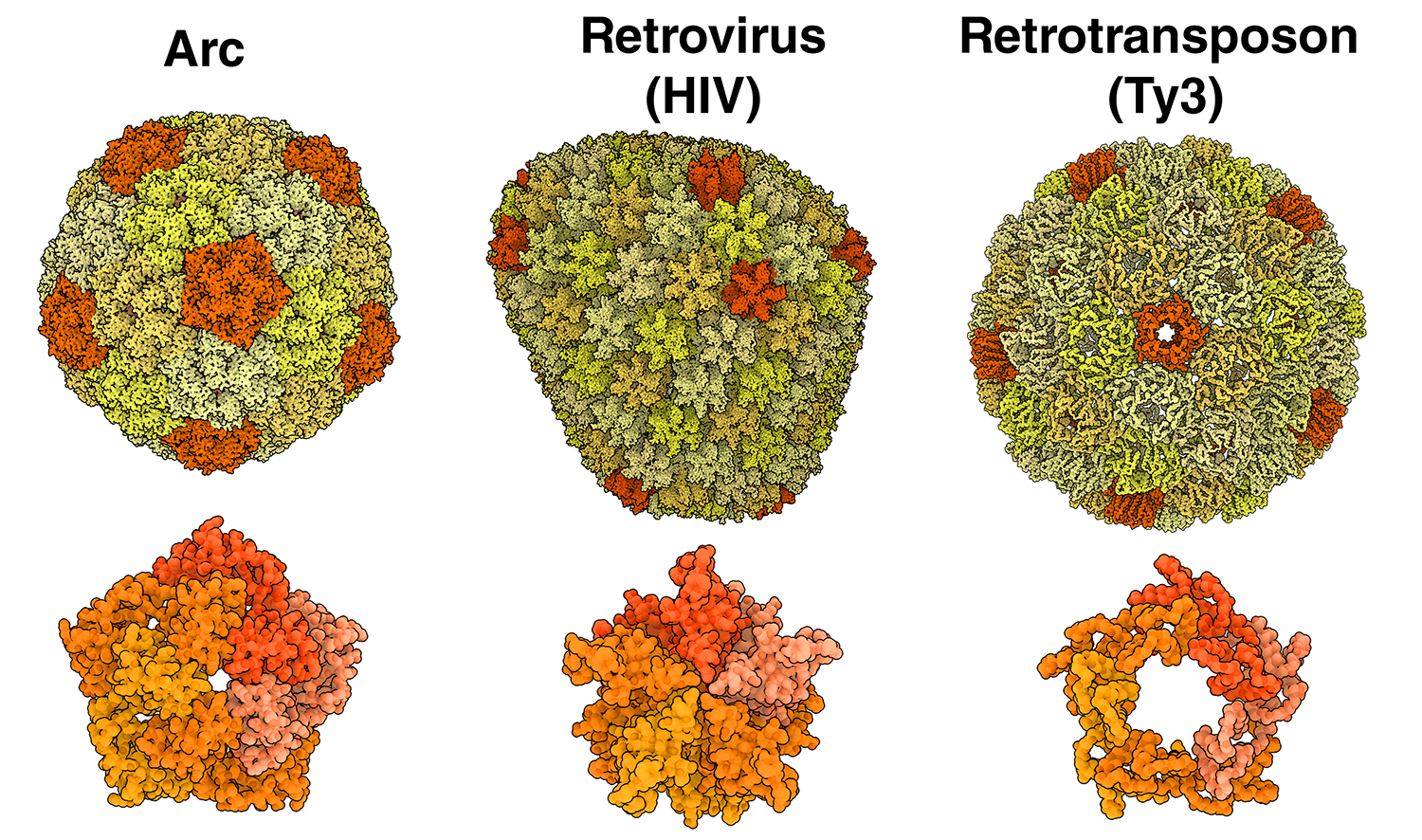
A shared history among Arc, retroviruses, and retrotransposons
Exploring the Structure
Compare the structure of an Arc capsid pentamer with a retrovirus and a retrotransposon
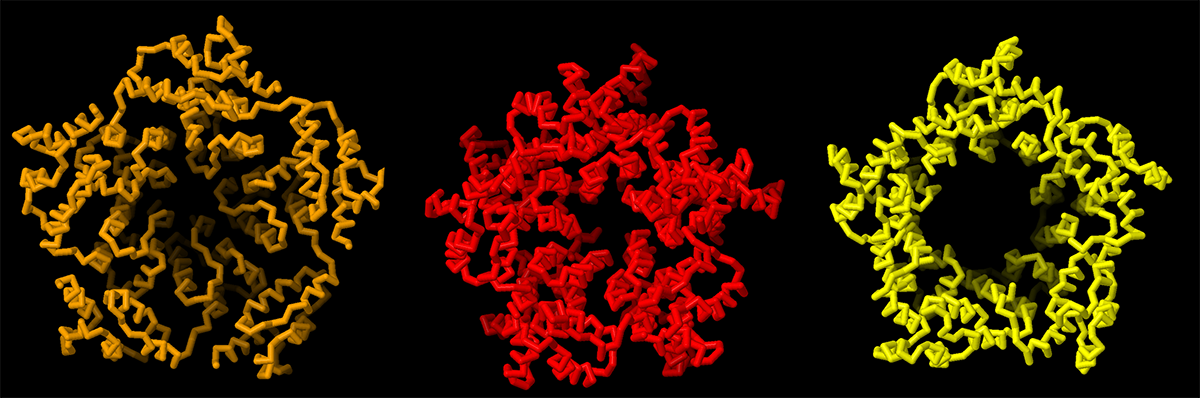
Arc, retroviruses and retrotransposons can all build capsids made up of CA proteins that form an arrangment of pentamers and hexamers. Take a closer look at pentamers from dArc1 (orange, 6TAR), the retrovirus HIV (red, 7URN), and the retrotransposon Ty3 (yellow, 6R24).
Topics for Further Discussion
- Transposons (or jumping genes) utilize enzymes known as transposases to excise a piece of DNA and move it to a different location. Learn more about transposases.
- Learn more about the retrovirus HIV and AIDS and HIV capsid.
Related PDB-101 Resources
- Browse Biomolecular Structural Biology
- Browse Molecular Evolution
- Browse Viruses
- Browse Drugs and the Brain
References
- 6TAP, 6TAR: Erlendsson S, Morado DR, Cullen HB, Feschotte C, Shepherd JD, Briggs JAG. Structures of virus-like capsids formed by the Drosophila neuronal Arc proteins. Nat Neurosci. 2020 Feb;23(2):172-175. doi: 10.1038/s41593-019-0569-y.
- 3J3Q: Zhao G, Perilla JR, Yufenyuy EL, Meng X, Chen B, Ning J, Ahn J, Gronenborn AM, Schulten K, Aiken C, Zhang P. Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics. Nature. 2013 May 30;497(7451):643-6.
- 7URN: Schirra RT, Dos Santos NFB, Zadrozny KK, Kucharska I, Ganser-Pornillos BK, Pornillos O. A molecular switch modulates assembly and host factor binding of the HIV-1 capsid. Nat Struct Mol Biol. 2023 Mar;30(3):383-390.
- 6R24: Dodonova SO, Prinz S, Bilanchone V, Sandmeyer S, Briggs JAG. Structure of the Ty3/Gypsy retrotransposon capsid and the evolution of retroviruses. Proc Natl Acad Sci U S A. 2019 May 14;116(20):10048-10057.
- Zhang W, Wu J, Ward MD, Yang S, Chuang YA, Xiao M, Li R, Leahy DJ, Worley PF. Structural basis of arc binding to synaptic proteins: implications for cognitive disease. Neuron. 2015 Apr 22;86(2):490-500.
- Pastuzyn ED, Day CE, Kearns RB, Kyrke-Smith M, Taibi AV, McCormick J, Yoder N, Belnap DM, Erlendsson S, Morado DR, Briggs JAG, Feschotte C, Shepherd JD. The Neuronal Gene Arc Encodes a Repurposed Retrotransposon Gag Protein that Mediates Intercellular RNA Transfer. Cell. 2018 Mar 22;173(1):275.
August 2025, Janet Iwasa
http://doi.org/10.2210/rcsb_pdb/mom_2025_8


